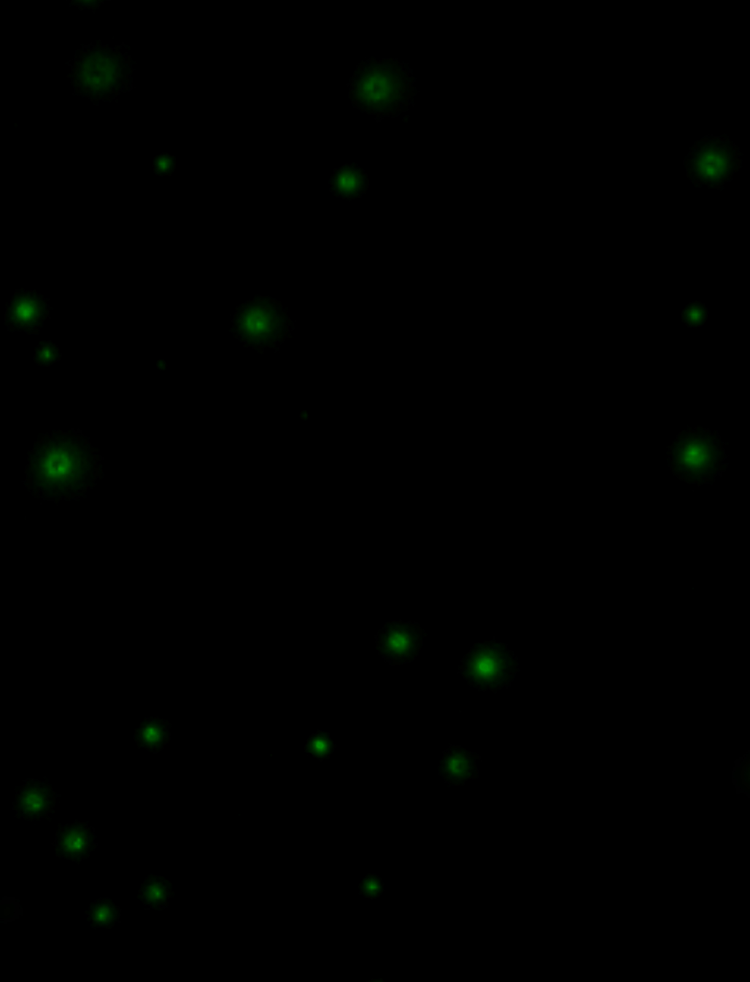
Criblage de clones
Solutions spécialisées pour le criblage de clones et l’isolation de cellules uniques
- Probabilité accrue de découverte de clones d’intérêt rares
- Sélection objective de clones de mammifères et de colonies microbiennes
- Techniques éprouvées permettant d’établir la monoclonalité
Systèmes de criblage de clones pour la vérification de la monoclonalité et la sélection de colonies
Nos solutions de détection d’anticorps et de développement de lignées cellulaires offrent des produits spécialisés, évolutifs et faciles à utiliser permettant d’établir des populations de clones. Ces systèmes offrent une sélection complète d’options et de modèles adaptés à vos recherches spécifiques, notamment plusieurs modes d’imagerie, des pointes spécialisées pour la biologie, des systèmes fluidiques et le contrôle environnemental. Ces solutions associent l’imagerie intelligente à l’analyse et à l’automatisation pour augmenter le débit et la régularité et pour fournir des documents basés sur l'imagerie.

Vérification facile de la monoclonalité
La sélection objective, l’imagerie et le recueil de données optimisent le suivi de la formation des colonies à partir d’une seule cellule.

Tri efficace des cellules uniques viables
L’association d’une robotique de haute précision à des systèmes fluidiques légers permet d’établir des clones viables de manière beaucoup plus efficace.

Flux de travail optimal
L’automatisation avec suivi des échantillons augmente le débit, permet davantage de temps sans surveillance et fournit des résultats uniformes.
https://share.vidyard.com/watch/2dDXoDw8pL2dHFbYC2jdoQ
Présentation du distributeur de cellules uniques DispenCell
Série QPix 400
Automatisez complètement vos protocoles de biologie de synthèse pour l’assemblage d’ADN, la détection d’anticorps et l’ingénierie des protéines
Les systèmes de repiquage de colonies microbiennes QPix® 400 Series associent l’analyse intelligente des images à une automatisation précise pour un criblage rapide et efficace de grandes bibliothèques. Proposant une gamme d'outil pour vos tests et pour le suivi de vos données, le logiciel QPix optimise le contrôle et la gestion des processus complexes et itératifs.
- Automatisez plusieurs processus de préparation d’échantillons et de manipulation de plaques, notamment le transfert des cultures bactériennes liquides et l’ensemencement sur gel d'agarose
- Optimisez votre flux de travail grâce à l’automatisation évolutive : sélectionnez jusqu’à 30 000 colonies par jour
- Suivi électronique des données pour un contrôle des données bien documenté
- Environnement stérile avec options de filtration HEPA sur mesure
Série ClonePix
Automatisez vos protocoles de détection d’anticorps et de développement de lignées cellulaires
Grâce à la série ClonePix®, criblez davantage de clones en moins de temps, avec la vérification de la monoclonalité au jour 0, puis criblez et sélectionnez les producteurs les plus performants en quelques semaines, et non en quelques mois.

- Criblez 10 fois plus de clones qu’avec une dilution limitante
- Augmentez la probabilité d’identifier des clones de grande valeur
- Condensez votre flux de travail en une solution unique
- Éliminez ou récupérez les clones instables de façon précoce
CloneSelect Imager et CloneSelect Imager FL
Vérifiez la monoclonalité en toute confiance
Le tout nouveau CloneSelect Imager FL ajoute une technologie fluorescente multicanaux haut contraste en plus de l’imagerie standard en lumière blanche qui permet de détecter avec précision les cellules uniques et de prouver la monoclonalité dès le premier jour. Simplifiez votre séquence de travail grâce à des tests de confluence comparatifs pour identifier et vérifier les modifications des gènes.

- Documentez de façon numérique les preuves des cellules uniques et de la confluence pour les audits et la soumission aux organismes de réglementation
- Capturez les cellules de façon non invasive à plusieurs points de capture dans le temps afin de surveiller la formation des colonies
- Criblage par imagerie en lumière blanche haute résolution
- Obtenez des résultats en temps réel grâce à une analyse à la volée
- Automatisation et intégration prêtes
Distributeur de cellules uniques DispenCell™
Un distributeur simple de cellules uniques pour la preuve de monoclonalité
Le distributeur de cellules uniques DispenCell est un instrument de laboratoire automatisé conçu pour une isolation rapide, facile et délicate des cellules uniques. La technologie de distribution des cellules permet aux chercheurs d’isoler des lignées cellulaires uniques trois fois plus rapidement et à moindre coût par rapport aux solutions existantes.

- Paramétrage simple et intuitif sans nettoyage ni étalonnage nécessaire
- Logiciel fournissant une preuve instantanée de clonalité et une traçabilité après la distribution des cellules
- La technologie unique traite délicatement l’échantillon cellulaire pour une meilleure viabilité et une plus grande efficacité de clonage par rapport au pipetage manuel
- Format plan de travail conçu pour s’adapter sous une hotte, sur un plan de travail ou dans un flux de travail automatisé préexistant
- Une pointe jetable brevetée pour garantir l’isolation propre des cellules uniques et l’absence de contamination croisée, certifiée sans produits d’origine animale ni matériel cytotoxique

Inscripta vous permet de réaliser une édition génomique numérique grâce à son système Onyx intégré dans un flux de travail totalement automatisé incluant le système QPix

Le Fred Hutchinson Cancer Research Center utilise le système ClonePix 2 pour cribler et isoler efficacement des clones d’hybridomes positifs
L’École de Médecine d’Harvard utilise des systèmes de repiquage de colonies QPix pour cartographier les réseaux d’interactions macromoléculaires afin de mieux comprendre la relation entre les gènes et les phénotypes

Zymergen utilise les systèmes de repiquage de colonies QPix pour améliorer la production de microbes utilisés dans la fermentation industrielle

L’Université d’Édimbourg utilise des systèmes de repiquage de colonies QPix pour accroître sa production d’ADN
Applications et recherche
Trouvez de nombreuses notes d’application, recherches et flux de travail relatifs au criblage de clones, notamment le développement de lignées cellulaires, la détection d’anticorps, et bien plus encore.
Développement de lignées cellulaires
Les lignées cellulaires stables sont largement utilisées dans de nombreuses applications importantes, notamment les agents biologiques (p. ex., ...
Découverte et optimisation d’antigènes/immunogènes
Les flux de travail du développement d’un vaccin varient largement selon la plateforme (p. ex., virus inactivé vs. vaccin à base d’ADN)…
Découverte d’anticorps en utilisant l'expression des phages
L'affichage de phage est une technique utilisée pour étudier l’interaction des protéines qui s’affichent à la surface d’un bactériophage avec d’autres…
Flux de travail d’hybridome pour la découverte d’anticorps
La technologie d’hybridome est une méthode pour la production en masse d’anticorps dans une lignée cellulaire hybride générée à partir de la fusion…
Monoclonalité
La monoclonalité est un terme décrivant une lignée cellulaire issue d’un progéniteur unique (cellule unique), et qui est donc monoclonale. Le développement de lignées cellulaires et l’assurance de monoclonalité constituent des étapes essentielles du processus de génération de molécules biopharmaceutiques, telles que les anticorps monoclonaux.
Anticorps monoclonaux (mAb)
Les anticorps monoclonaux (mAb) sont issus d’une cellule parent unique et ne se lient donc qu’à un seul épitope. La détection d’anticorps monoclonaux renvoie généralement au criblage et à l’identification d’anticorps spécifiques qui ciblent un épitope spécifique pour le diagnostic et le traitement de maladies, comme le coronavirus pour la COVID-19.
Biologie de synthèse
La biologie de synthèse est un terme général qui fait référence à la manipulation de voies génétiques pour exploiter la puissance de systèmes biologiques existants de façon novatrice (souvent pour fabriquer des molécules ou des protéines). La biologie de synthèse applique des principes qui dérivent de l’ingénierie, spécifiquement des cycles de « conception-fabrication-mise à l’essai-apprentissage », à des systèmes biologiques. En utilisant des protocoles haut débit, les biologistes de synthèse peuvent accélérer ce processus.

Automatisation de laboratoire pour le criblage de clones haut débit
Les projets de criblage de clones de mammifères et de microbes commencent généralement par une cible, un récepteur, une protéine ou un gène qui participe à une voie biologique d’intérêt. Vient ensuite le criblage, où des milliers ou des millions de cellules sont testées et analysées par rapport à la cible. Cela représente un goulot d’étranglement important pour les laboratoires, car nécessitant des méthodes fastidieuses et longues sur un grand nombre de plateformes analytiques.
Édition génomique (CRISPR/Cas9)
L’édition génomique est une manipulation génétique au cours de laquelle l’ADN génomique d’un organisme vivant est supprimé, inséré, remplacé ou modifié. L’édition génomique est un ciblage spécifique à un site permettant de créer des cassures dans l’ADN par le biais de diverses techniques et ne fait pas toujours intervenir les mécanismes de réparation.
Produits et services connexes
Découvrez notre gamme complète de systèmes de criblage de clones et notre gamme d’appareils de laboratoire et de milieux haute performance. Nous proposons également des services de personnalisation et de robotisation pour les instruments.

Milieu
Une gamme de milieux haute performance. L’utilisation de CloneMedia garantit la formation de colonies discrètes de clones avec des lignées cellulaires telles que les hybridomes, les CHO, CHO-S, CHOK1 et d'autres.
Consommables
Assortiment d’appareils de laboratoire de haute performance, dont cartouches, modules, filtres et microplaques, pour n’en nommer que quelques uns.

Services
Personnalisez vos instruments et automatisez l’intégralité de vos flux de travail pour répondre aux besoins particuliers de vos tests, méthodes ou protocoles
Comment pouvons-nous vous aider à progresser vers votre prochaine grande découverte ?
Nos équipes hautement qualifiées sont en première ligne avec vous, réalisant des démonstrations des produits à distance ou sur site, des webinaires, et bien plus encore, pour vous aider à résoudre les défis liées à vos recherches. Comment pouvons-nous vous aider aujourd’hui ?
Je souhaiterais…