
Application Note
NanoBRET Technology on the SpectraMax i3x Multi-Mode Microplate Reader
- Study induction and inhibition of protein interactions with a physiologically relevant system
- Achieve increased signal and lower background compared to conventional BRET assays
- NanoBRET detection module for SpectraMax i3x offers extra sensitivity for assays that require it
Cathy Olsen, PhD | Sr. Applications Scientist | Molecular Devices
Introduction
Protein-protein interactions (PPI) are vital to cellular function and signaling. Many methods have been employed to study PPI, including co-immunoprecipitation and pull-down assays, as well as fluorescence methods such as fluorescence resonance energy transfer (FRET). Because they rely on excitation of a sample by a laser or high-powered lamp, fluorescence methods are often prone to issues of photo-bleaching and background fluorescence, which can limit their usefulness. An alternative method, bioluminescence resonance energy transfer (BRET), uses a chemical reaction catalyzed by the enzyme luciferase to generate light. Because there is no optical excitation, BRET avoids problems posed by sample illumination with an external light source.
NanoBRET™ Technology, from Promega, uses NanoLuc® Luciferase as the BRET donor and HaloTag® protein labeled with the NanoBRET 618 fluorophore as acceptor. The red-shifted acceptor reduces overlap of the donor and acceptor emission spectra, enabling better signal to noise than other BRET methods that use Renilla luciferase as a donor and YFP or GFP as acceptor.
Here, we demonstrate validation of the SpectraMax® i3x Multi-Mode Microplate Reader for NanoBRET assays. The onboard luminescence detection mode may be used, or, for higher sensitivity, the reader can be equipped with a NanoBRET detection cartridge that offers a 4-fold improvement in limit of quantitation.
Materials
- White 96-well plate
- 1X phosphate-buffered saline (PBS) containing 0.1% bovine serum albumin (BSA)
- NanoBRET Control protein panel – 5 vials representing the following amounts of fractional occupancy:
- NanoBRET Control Protein 1: 0%
- NanoBRET Control Protein 2: 0.1%
- NanoBRET Control Protein 3: 1%
- NanoBRET Control Protein 4: 10%
- NanoBRET Control Protein 5: 100%
- NanoBRET Nano-Glo Substrate (part of NanoBRET Detection System)
- SpectraMax i3x Multi-Mode Microplate Reader
- NanoBRET detection cartridge
Methods
An in vitro protocol provided by Promega for using a NanoBRET control protein was run to validate instrument setup and estimate the detection limit. A series of standards containing increasing amounts of NanoBRET active protein were plated in a 96-well solid white microplate for NanoBRET measurements. These measurements were used to generate linear regression calculations and limit of quantitation (LOQ) values used to gauge instrument performance.
The validation method was run on the SpectraMax i3x reader using either a custom NanoBRET detection cartridge or the onboard luminescence detection optics. Results for both are presented.
Assay method
- Dispense 50 µL of control protein into triplicate wells of a 96-well plate.
- Prepare a 20 µM solution of NanoBRET Nano-Glo Substrate in 1X PBS/0.1% BSA (250-fold dilution of 5 mM stock reagent).
- Add 50 µL of substrate to each well for a final concentration of 10 µM.
- Within 10 minutes of substrate addition, measure donor emission (e.g. 450 nm) and acceptor emission (e.g. 610 nm) using a NanoBRET-compatible luminometer
Data analysis
- Generate NanoBRET ratios using the following formula: Acceptor sample /Donor sample .
- Determine average NanoBRET ratio and SD for each set of data: 0%, 0.1%, 1%, 10%, and 100%.
- Subtract the average ratio for the 0% set from all other sets to obtain the corrected NanoBRET ratio, which represents the value for true energy transfer.
- Plot the corrected NanoBRET ratios and perform linear regression analysis in order to calculate the slope. The curve should be forced through x,y = 0 (since no BRET is possible in the absence of acceptor).
- Calculate the limit of quantitation (LOQ), which represents the minimum percentage of BRET pairs relative to the total donor population (fractional occupancy) that can be statistically distinguished from donor alone: LOQ = (10 * SD 0% sample )/slope.
Results/Conclusion
The LOQ for the SpectraMax i3x reader using the NanoBRET detection module was 0.58% fractional occupancy (Figure 1). Using the onboard luminescence detection mode, the LOQ was 2.6%. The higher sensitivity obtained with the NanoBRET detection module may benefit assays where signal is lower, while the onboard luminescence detection will be sufficient for many assays with stronger signal.
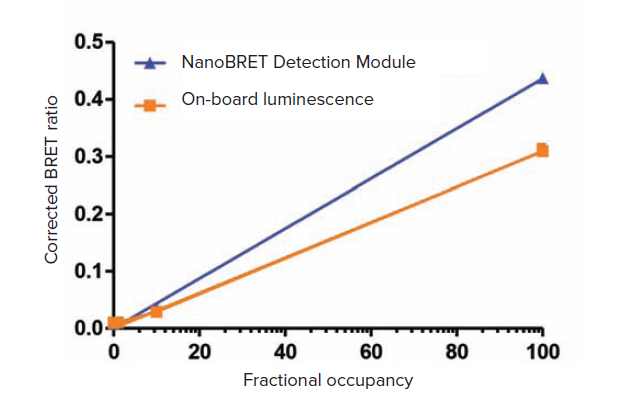
Figure 1. Results for SpectraMax i3x reader with NanoBRET Detection Module (blue) and on-board luminescence detection mode (orange). Curves were forced through x,y = 0. The calculated LOQ values were 0.58% for the detection module and 2.6% for the on-board optics (monochromators).
简介
研究蛋白质之间的相互作用 (PPI) 对于探索细 胞功能及信号转导通路来说至关重要,目前有 许多种方法来研究蛋白质之间的相互作用。 其中包括免疫共沉淀技术,以及基于荧光基 础理论的荧光共振能量转移技术 (FRET),由 于基于荧光的方法依赖于激光或高能氙闪灯 作为光源来对样品的进行激发,因此往往容 易产生光漂白和背景荧光等问题,从而大大 限制了基于荧光方法的实用性。一种可以替 代的方法,生物荧光共振能量转移 (BRET), 是利用荧光素酶催化的化学反应来产生光学 信号,由于这种方法无需外置光源激发作用, BRET 可以有效避免由于外来光源激发带来 的种种弊端。
来自于 Promega 的 Nano BRET 技术,其利 用了 Nano®Luc 荧光素酶作为 BRET 检测供 体分子,标记有 HaloTag® 荧光基团的蛋白 发射 618 nm 荧光波长作为受体分子。发生 红移现象的受体分子减少了供体和受主荧光 信 光 谱 的 光 谱 重 叠 作 用 , 较 替 他 类 型 的 BRET 方法具有更好信噪比,如 Renilla 荧光 素酶为供体,YFP 或GFP 作为受体的 BRET1 和 BRET2。
本篇技术性短文介绍了如何利用 SpectraMax i3x 多功能微孔板读板机进行 NanoBR ET 检 测,分别介绍了如何利用仪器内置的检测模 式进行 NanoBRET 检测以及如何利用具有更 高灵敏度的 NanoBRET 卡盒进行相应检测, 如果利用更灵敏的检测卡盒可将定量检测下 限提高四倍以上。
材料
- 白色 96 孔板
- 1XPBS 缓冲液 ( 包括 0.1BSA )
- NanoBRET 质控蛋白-5 瓶的含量分别如下
- NanoBRET 质控蛋白 1:0%
- NanoBRET 质控蛋白 2:0.1%
- NanoBRET 质控蛋白 3:1%
- NanoBRET质控蛋白4:10%
- NanoBRET质控蛋白5:100%
- NanoBRET Nano-Glo 底物 ( NanoBRET 检 测体系 )
- SpectraMax i3x 多功能微孔板读板机
- NanoBRET 检测卡盒
验证方法
利用 Promega 提供的一种用 NanoBRET 质 控蛋白检测体系的实验方案来优化仪器设置 并探索其不同条件下的检测下限,将梯度浓 度稀释的具有 NanoBRET 活性的蛋白标准品 固定在白色 96 孔板上用于相应的检测,这 些测量值用于生成线性回归计算和定量其检 测下限 (LOQ) 值,可以测量仪器性能。
此验证方案使用 SpectraMax i3x 微孔板读板 机内置化学发光检测模式或专用 NanoBRET 检测卡盒分别进行检测获得结果。
检测方法
- 将 50 µl 质控蛋白分别以 3 个复孔方式加入 96 孔板中
- 准备 20 µM 的 1XPBS/0.1% BSA 的 NanoBRET 的 Nano-Glo 底物溶液 ( 5 nM 储存液以 250 倍的方式进行稀释)
- 加入 50 µl 底物溶液使之每个孔的终浓度为 10 µM
- 在底物加入的十分钟内进行相应检测,使 用具有 NanoBRET 检测功能的仪器来分别 检测供体发射光强度 (450 nm) 和受体分子 发射光强度 (610 nm)
数据分析
- 产生的 NanoBRET 信号比率,即受体分子 信号/供体分子信号
- 确定 NanoBRET 检测每组数据的平均比率 和相对标准偏差
- 所有其他数据组平均比率值减去 0% 组数据 的平均比率值,获得校正过的 NanoBRRT 值,代表了真正的能力转移信号值
- 绘制校正获得 NanoBRET 比率值曲线并选 择线性回归方式进行拟合,计算曲线斜率, 曲线应该强制 X,Y = 0 ( 因为没有检测到受 体分子信号强度的话 NanoBRET 现象就不 会产生 )
- 计算定量下限 (LOQ),哪个代表了 BRET 配 对相对于总供体 ( 部分占用率 ) 的最小百分 比,可以从统计学上与单独供体区分开, LOQ = ( 10*SD 0% 样品 ) /斜率。
结果
SpectraMax i3x 多功能微孔板断布机进行 NanoBRET 的检测专用模块情况下,LOQ 为 0.58%,使用内置的发光检测模块情况下可 达到 LOQ 为2.6%,专用检测模块可以提升 微弱限号检测灵敏度和动态学范围,仪器内 置化学发光检测模块更适用于信号比较强的 实验。

图 1 SpectraMax i3x 微孔板读板机进行 NanoBRET 检测情况下,分别使用内置发光检测模块或 者专用NanoBRET检测卡盒模块获得数据。 曲线绘制强制其 X,Y = 0,专用外置检测模块下和内置 检测模块 ( 内置四光栅系统 ) 下分别计算的 LOQ 为 0.58% 和 2.6%