
Application Note
High-Throughput Confocal Imaging of Spheroids for Screening Cancer Therapeutics
- Capture an entire spheroid in one field-of-view at 20X magnification
- Screen biologically relevant 3D spheroids in a 96- or 384- well format
- Use confocal imaging to accurately detect cellular responses
- Conserve storage space by saving only 2D reconstructions of the z plane images
Automated high-throughput, high-content imaging allows for rapid acquisition and analysis of 3D spheroids
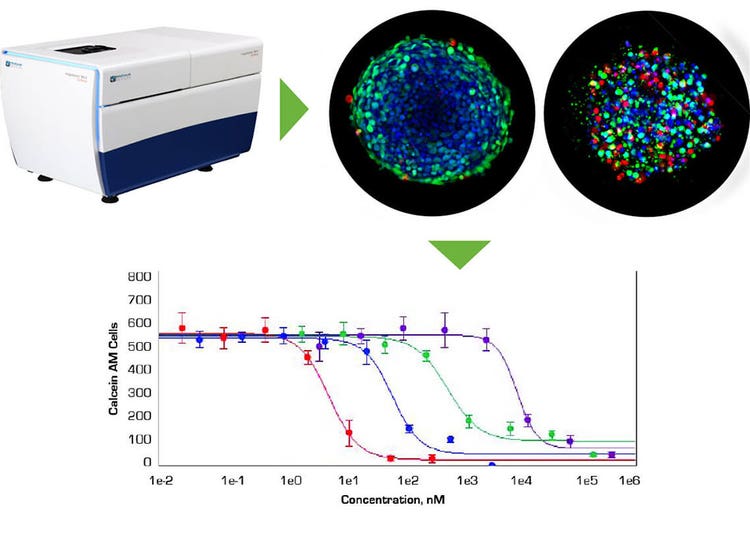
The ability of in vivo 3D culture systems to produce human cancer cell spheroids of uniform size and the ability to screen spheroid response to treatment using automated high-throughput, high-content imaging is a significant step in facilitating more relevant testing of chemotherapeutic drug candidates. The ImageXpress Micro Confocal system and MetaXpress software allow rapid imaging and analysis of 3D spheroids in microplates for monitoring induced apoptosis and mitochondrial toxicity of anti-cancer drugs.
- Introduction
- Spheroid formation and treatment
- Staining and imaging spheroids
- ImageXpress Micro Confocal system generates sharper images for more accurate segmentation
- Screening anti-cancer drugs with an apoptosis assay
- Multiplexing a mitochondria membrane potential assay in the screen
- Rapidly screen 3D spheroids in microplates
Introduction
In recent years, there has been significant progress in development of in vitro aggregates of tumor cells for use as models for in vivo tissue environments. When seeded into a well of a lowattachment round bottom microplate, these aggregates will form a discrete spheroid. Spheroids are believed to mimic tumor behavior more effectively than regular two dimensional (2D) cell cultures because, much like tumors, they contain both surface-exposed and deeply buried cells, proliferating and non-proliferating cells, and a hypoxic center with a well-oxygenated outer layer of cells. Such 3D spheroid models are being successfully used in screening environments for identifying potential cancer therapeutics. Some challenges to developing robust spheroid assays:
- Locating and focusing on the spheroid in every well so it can be imaged in a single field of view
- Optimizing the compound and staining treatment to ensure dye penetration and avoid disturbing the spheroid placement
- Acquiring representative images throughout the 3D structure, minimizing out-of-focus or background signal from above and below the imaging plane
- Rapidly analyzing the images to yield meaningful results from which conclusions can be drawn
Spheroid formation and treatment
We used the following method to form spheroids from cancer cell lines HCT116, DU145, and HepG2. Cells were cultured in flasks at 37°C and 5% CO2 before detaching and seeding into 96- or 384-well black plates with clear bottom U-shaped wells (Corning 4520 and 3830, respectively) at densities of 1000- 1500 cells/well in the appropriate media supplemented with fetal bovine serum (FBS). Within 24 hours, a single spheroid formed in the bottom of each well and continued growing in size until it was used for experimentation after 2-4 days at 37°C and 5% CO2 (Figure 1). Spheroids may be cultured longer but the increasing size may impede stain penetration and imaging of the center-most cells. This application note describes assays used to determine the effects of the anti-cancer compounds: etoposide, paclitaxel, and Mitomycin C. Spheroid treatment began by adding compounds into the wells at 10x concentration then incubating for 1-4 days, depending on the mechanism being studied. Shorter durations were used to study apoptosis and longer durations for multi-parameter cytotoxicity studies. For drug treatments longer than 2 days, compound was refreshed every 2 days at a 1x concentration.
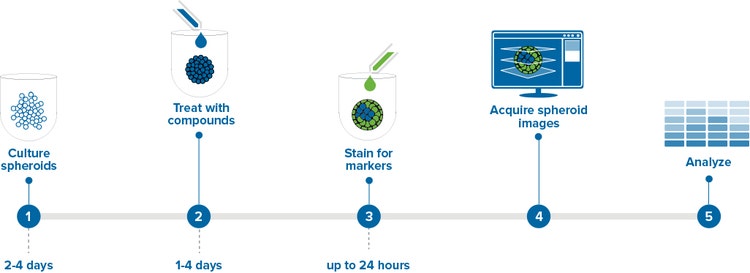
Staining and imaging spheroids
The examples shown here are from the development of an HCT116 spheroid assay for evaluating spheroid morphological changes in addition to the incidence of apoptotic cells in the well. After the compound treatment was completed, stains were combined into a single cocktail at 4-6x concentration and added directly to the media in the wells. Stains that require no washing were chosen to avoid disturbing the spheroids.
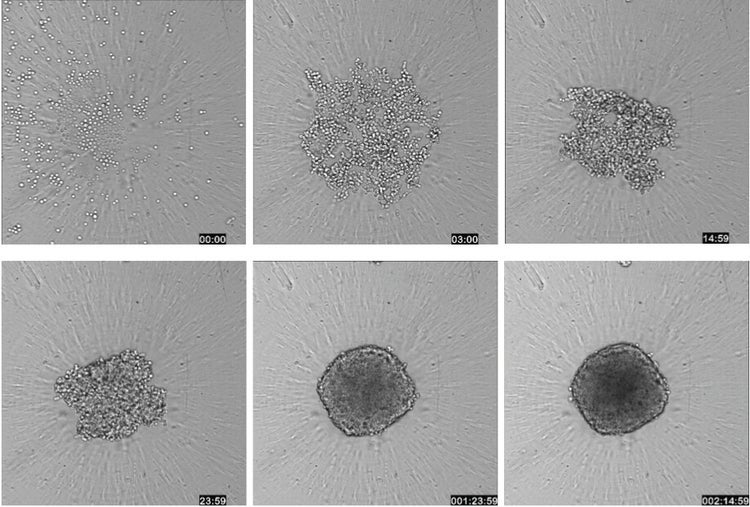
Figure 1: A workflow for testing spheroids in a high-throughput screening environment. A single spheroid can be grown in a 96- or 384-well plate, treated with compound, and stained with a cocktail of dyes that can be imaged without washing. Spheroids may also be fixed if desired. (Right) Transmitted light images of HCT116 cells were taken over the course of 63 hours using Timelapse acquisition on ImageXpress Micro confocal System to show the formation of a spheroid (10X objective).
Spheroids were visualized using the ImageXpress® Micro Confocal High-Content Imaging System at either 10X or 20X magnification. In order to analyze responses of cells throughout the 3D structure, images were collected from different depths within the body of the spheroid to create a “stack” of images. That stack of images was then combined or “collapsed” into a single 2D projection image using a mathematical algorithm. In this case a collapsed image was generated using the MetaXpress® High-Content Image Acquisition and Analysis Software's Maximum Projection algorithm, which keeps the pixels with the brightest intensity in the stack to generate the projection (Figure 2).

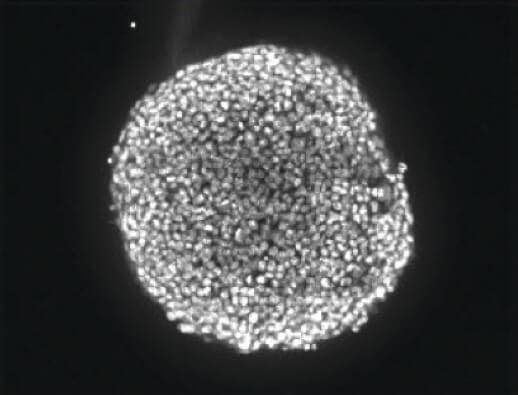
Figure 2: A stack of confocal images was acquired in the z plane spanning roughly half the depth of the spheroid (left). Only some cells of the spheroid are in focus at any given plane so, for ease of analysis, the images were collapsed into a single 2D image to combine the in-focus areas (right).
ImageXpress confocal system generates sharper images for more accurate segmentation
Confocal optics provide the ability to image a thinner optical section of the spheroid than widefield optics. This significantly reduces the amount of background haze produced by fluorescence-emitting objects above and below the plane being acquired. It also allows better resolution of fine detail either at the subcellular level or between cells that are clustered or stacked upon each other as they are within a 3D structure. More accurate segmentation is often possible using a confocal image. In repeated experiments with spheroids, segmentation of nuclei from widefield images yielded counts \~20% lower than nuclei counted in confocal images (Figure 3).
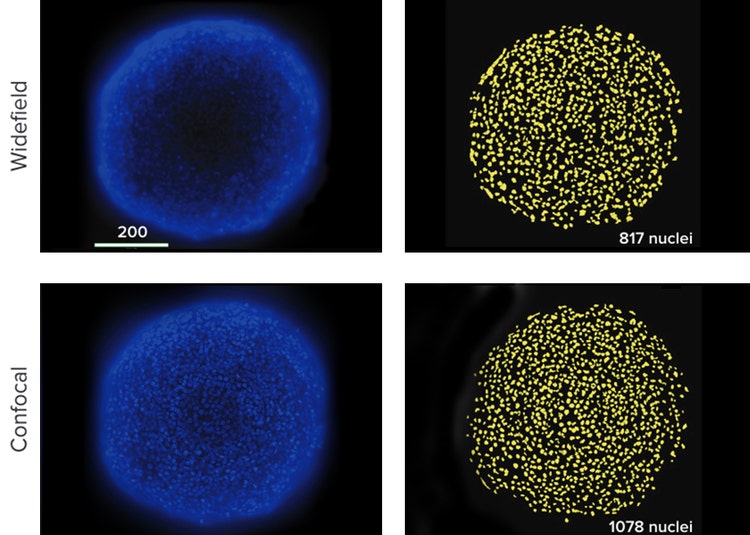
Figure 3: (Top) Best focus projection of 15 images of an HCT116 spheroid taken with widefield optics. Software segmentation counted 817 nuclei. Nuclei were missed due to distortion by unfocused fluorescence on the edges of the spheroid and poorly detected dim cells in the center. (Bottom) Best focus projection of 15 images of an HCT116 spheroid taken with confocal optics. A more accurate number, 1078 nuclei, was counted.
Screening anti-cancer drugs with an apoptosis assay
One class of anti-cancer drugs targets the extrinsic pathway of apoptosis to trigger cell death. To demonstrate an assay for apoptosis, HCT116 spheroids cultured in 96-well plates for 3 days were treated with a dilution series of 4 different anticancer compounds for 24-48 hours. After the compound treatment was completed, apoptosis was detected using both CellEvent Caspase and MitoTracker Orange reagents from Life Technologies. A 4x cocktail of the combined stains, including Hoechst nuclear stain, was added to the media in the wells. Stains that require no washing out were chosen to avoid disturbing the spheroids (Figure 4).
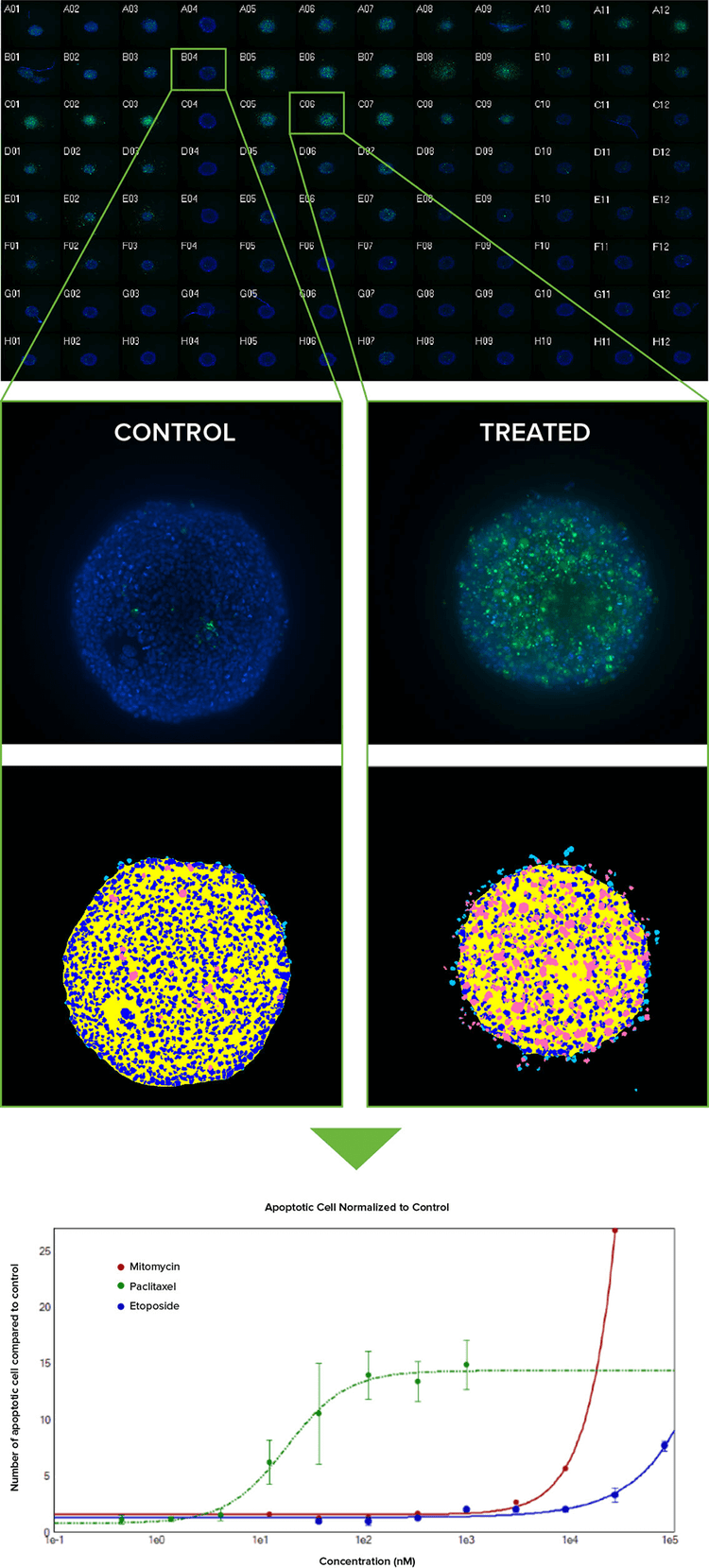
Figure 4: (Top) Montage of image thumbnails of HCT116 spheroids in a 96-well plate treated with compounds and imaged with a 10x Plan Fluor objective. Hoechst stained nuclei (blue) are overlaid with CellEvent Caspase 3/7 apoptosis marker (green). Untreated controls are in column 4 and a Caspase 3/7 response is evident in columns 5–7 where Paclitaxel was serially diluted 1:3 from 1 uM in Row A (replicates of 3 across). (Middle) Eleven z planes were combined into a 2D Maximum Projection image and analyzed with a simple custom module. Raw images showing low and high degree of apoptosis with their corresponding segmentation masks are shown (royal blue = nuclei, pink = apoptotic cells). (Bottom) By normalizing the amount of apoptosis as compared to untreated spheroids and plotting on a graph, it can be seen that Paclitaxel (green line) induces apoptosis at a much lower concentration than either Mitomycin C or Etoposide.
Multiplexing a mitochondria membrane potential assay in the screen
In the apoptosis screen above, mitochondrial membrane potential can also be evaluated by adding MitoTracker Orange to the dye cocktail. Alternatively, drugs that inhibit tumor growth by affecting mitochondria metabolism can be studied separately. The following demonstrates an assay using Antimycin A, a potent disruptor of mitochondrial membrane potential. After four-hour treatment, mitochondria health was detectable based on the intensity of MitoTracker Orange within the spheroid cells. The MitoTracker either did not penetrate completely to the center of the large spheroids or the cells in the center do not have healthy mitochondria as noted by the interior appearing generally dimmer in the mitochondria wavelength in the images (Figure 5).
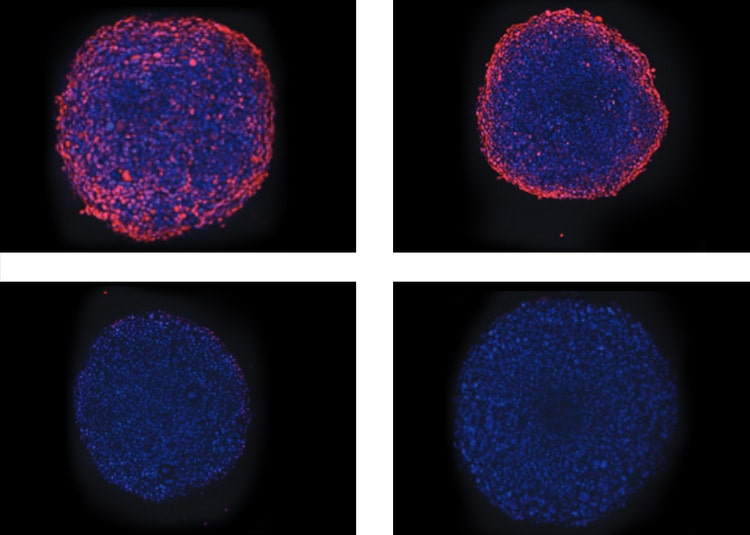
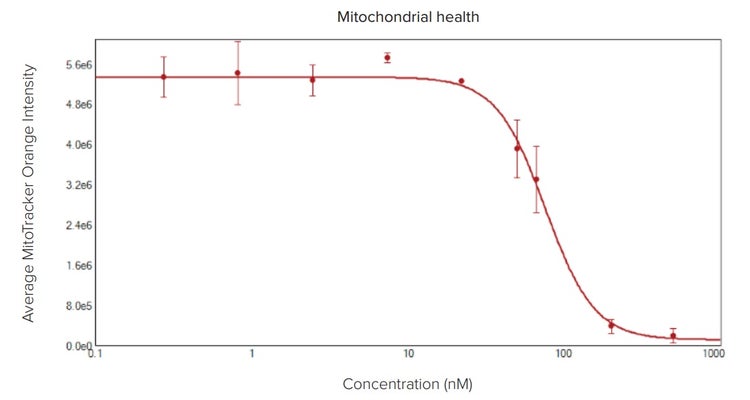
Figure 5: Toxic effect of Antimycin A on mitochondria. (Top) Overlay of Hoechst (blue) and MitoTracker (orange) images of spheroids treated with Antimycin A in increasing concentrations of 1, 22, 67, and 200 nM. (Bottom) Plotted average intensity values of mitochondria identified within the spheroid illustrate the effect of the drug.
Rapidly screen 3D spheroids in microplates
The ability of in vivo 3D culture systems to produce human cancer cell spheroids of uniform size and the ability to screen spheroid response to treatment using automated high-throughput, high-content imaging is a significant step in facilitating more relevant testing of chemotherapeutic drug candidates. The ImageXpress Micro Confocal System and MetaXpress Software allow rapid imaging and analysis of 3D spheroids in microplates for monitoring induced apoptosis and mitochondrial toxicity of anti-cancer drugs.
For further information on optimizing acquisition parameters of these spheroid screening assays, refer to the paper: Sirenko, O. et al., High-Content Assays for Characterizing the Viability and Morphology of 3D Cancer Spheroid Cultures. Assay and Drug Development Technologies, 2015 In Press.