
Application Note
Transmitted light image analysis for cell count and assessment of cytotoxicity effects
- Segment and count objects using transmitted light imaging
- Monitor cell proliferation in transmitted light
- Evaluate cytotoxicity effects without dyes
- Assess the effects of anti-proliferative and cytotoxicity compounds
Oksana Sirenko | Research Scientist | Molecular Devices
Introduction
Numerous biological applications require monitoring of cell number, cell health, confluency, and proliferation during multiple time points without fluorescent markers or other labels. In particular, there is an increased need for reliable and efficient cell counting methods to assess a variety of cell responses when monitoring cells with transmitted light (TL) imaging
Materials
- CHO cells (ATCC P/N CRL-1985)
- HeLa cells (ATCC P/N CCL-2)
- HeLa media (Gibco, ThermoFisher Scientific)
- Hoechst (ThermoFisher Scientific)
- 96-well microplate (Greiner Bio-One International)
- ImageXpress® Nano Automated Imaging System with CellReporterXpress Automated Image Acquisition and Analysis Software (Molecular Devices)
Segment and count objects using transmitted light
CellReporterXpress software provides the ability to segment and count objects using transmitted light images. In the present study, we evaluated the accuracy of label-free cell counting by exploring the feasibility of transmitted light cell analysis for the assessment of proliferation and cytotoxic effects.
We evaluated the accuracy of cells counted in transmitted light using serial dilution and compared this to the value of cells counted using nuclear counterstain. CHO and HeLa cells were plated onto 96-well plates using the appropriate media (HeLa media: MEM + 10% FBS + 1% pen/strep; CHO media: Ham’s F12 + 10% FBS + 1% pen/strep). 20,000 cells/ well were seeded in the top row, and 50% serial dilutions were applied across the plate. 24 hours after plating, cells were stained with Hoechst nuclear dye, fixed with 4% paraformaldehyde, and washed with PBS. Images from individual wells were acquired with the ImageXpress Nano system using 10x or 4x objectives. One 10x image was captured per well in a 384-well plate. A 10x objective provides sufficient resolution while imaging a relatively large number of cells (500–1,000 cells per image) capturing about 1/4 of the total well area. One 4x image covers an entire 384-plate well (the equivalent of 1/8 of one well in a 96-well plate). Following image capture, all image analysis was accomplished using CellReporterXpress software, which contains a predefined image analysis protocol for quantifying cell count in transmitted light. Several analysis protocols are available for cells with different morphology. As an example of image processing, Figure 1 shows representative zoomed-in images of HeLa and CHO cells with the appropriate analysis masks
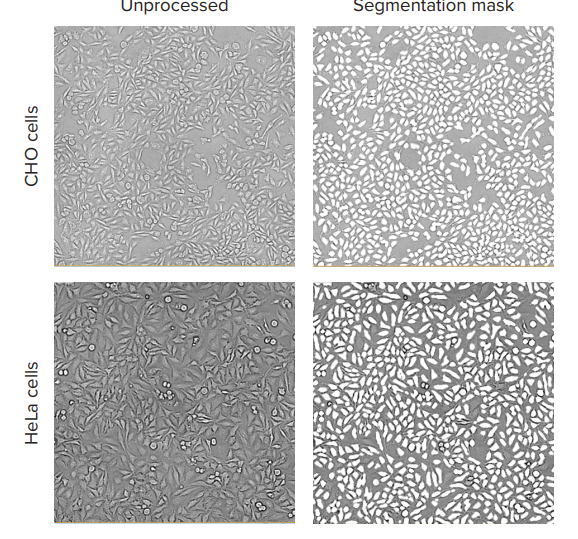
Figure 1. TL images and the software analysis masks shown for HeLa and CHO cell cultures. Images of CHO cells and HeLa cells were taken with the ImageXpress Nano system using a 10x Plan Fluor objective and transmitted light channel. Images were processed using CellReporterXpress software, TL Cell Count, General analysis protocol.
Typical exposure times for TL imaging, with 10x or 4x objectives, were 10-20ms. For better results, the focus offset was set in the range of -5 to -8 µm below the image-based autofocus. The predefined “TL Cell Count, General” analysis protocol accurately segmented cells at different cell densities for both HeLa and CHO cell lines (Figure 1). We compared cell counts obtained via TL imaging and analysis with standard cell counts using nuclear stain. The results of these two different methods of cell counting, via TL and fluorescent nuclear marker, demonstrate good concordance (Figure 2).
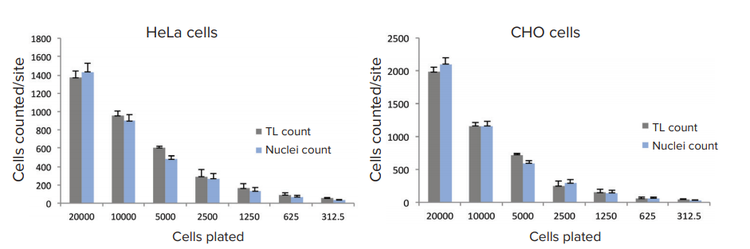
Figure 2. Comparison of cell counts based on TL analysis or nuclear count. HeLa and CHO cells were plated for 24h and were fixed and stained with Hoechst (16 µM). Images were acquired with transmitted light and DAPI using the ImageXpress Nano system with a 10x Plan Fluor objective. Images were processed using the TL Cell Count, General analysis protocol to count cells in transmitted light and using the standard Cell Count analysis protocol to count cells based on fluorescent nuclear stain.
Cytotoxic effects may be evaluated without fluorescently labeling cells
HeLa cells were treated with anti-cancer compounds mitomycin C, etoposide, paclitaxel, and doxorubicin for 72 h across a 6-point concentration range in 384-well plates (Figure 3). Live cells were imaged in transmitted light and analyzed using the “TL Cell Count, General” analysis protocol. Effects on cell proliferation and cell death were assessed by measuring cell number. There were significant decreases in the number of cells treated with indicated compounds. Concentration-responses were evaluated using a Hill model to derive EC50 concentration values. Cells treated with anti-cancer compounds demonstrated a clear decrease in cell numbers corresponding with increases in compound concentration (Figure 3).
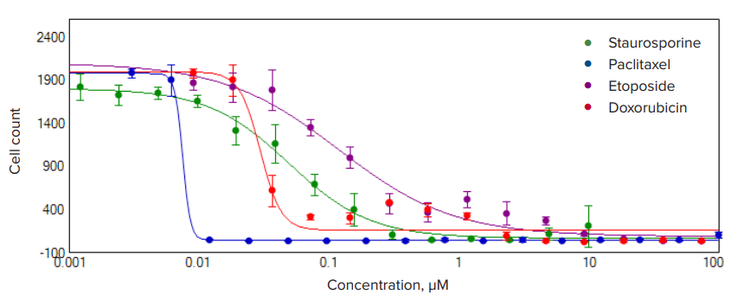
Figure 3. Concentration-response curves for four cytotoxic compounds. HeLa cells were plated into 384-well plates and treated with cytotoxic compounds for 72 hours. Images were acquired with the ImageXpress Nano system using transmitted light and DAPI, with a 10x Plan Fluor objective. Images were processed using TL Cell Count, General analysis protocol to count cells in transmitted light. The cell number decreases in a dose dependent manner for all compounds. A 4-parameter curve fit is shown for the selected compounds. Calculated EC50 values (nM) were the following: paclitaxel 8.0 ± 2.0; doxorubicin 30 ± 4; staurosporine 50 ± 5; and etoposide 121 ± 37.
Conclusion
The method demonstrates that TL analysis can be used for the accurate assessment of cell number as well as for measuring anti-proliferative and cytotoxicity effects of various compounds.