
Application Note
Detection and quantitation of protein with ScanLater Western Blot Detection System
- Western blot protocol is unchanged except for incubation with secondary antibody
- No substrates required
- TRF detection using Europium-labeled secondary antibody reduces background while increasing dynamic range
- Digital photon counting provides unaltered TRF counts
- Europium resists photo bleaching and is stable for weeks, enabling reuse of blots
Introduction
Protein detection is important for pharmaceutical and clinical research today, and western blots are among the most common methods employed for this purpose. Various techniques are used to detect proteins on western blot membranes including fluorescence and chemiluminescence. However, each technique has its limitations, and there is a continuing need to improve quantitation, accuracy, and dynamic range. Here we report on a novel system for western blot membrane protein analysis that is incorporated into SpectraMax® i3 and SpectraMax® Paradigm® Multi-Mode Microplate Readers. We demonstrate extended dynamic range due to reduced background as well as stability of detection over time and number of reads. In addition, a comparison of Scanlater Western Blot System to a chemiluminescence method demonstrates improved sensitivity.
Assay principle
ScanLater® Western Blot System workflow follows standard gel loading and blotting methods up to the secondary antibody incubation step. Membranes are incubated with Europium-chelate labeled secondary antibodies or streptavidin that bind specifically to the primary antibody bound to the protein of interest (Figure 1).
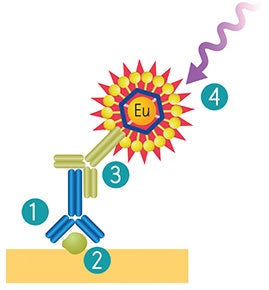
Figure 1. ScanLater Western Blot System workflow. Use existing primary antibody (1) for binding to protein of interest (2). Eu-labeled ScanLater Secondary Antibody (3) binds to primary antibody. Detection with ScanLater TRF Western Blot Detection Cartridge (4).
Images are generated utilizing time resolved fluorescence (TRF) mode detection of Europium (Eu) which has a 1 ms fluorescence lifetime. This significantly reduces background from auto-fluorescence or other sources of short lifetime emissions. There is no camera blooming which is often seen with chemiluminescence or standard fluorescence detection; thus the system provides sharp bands and superior image quality.
The method does not involve enzyme detection, and the Eu-chelates are resistant to photo bleaching. Therefore, the signal remains stable for weeks to months. This stability enables repeat reading of membranes allowing more accurate quantitation. The new ScanLater™ Western Blot Detection System is a simple, sensitive, and stable platform that provides excellent protein analysis capability in a multi-mode plate reader.
SoftMax Pro Software analysis of western blot data
All experiments were conducted using the ScanLater Western Blot Detection System for either the SpectraMax i3 or SpectraMax Paradigm Multi-Mode Microplate Reader. By using a microplate detection system, there are distinct advantages. First, digital photon counting provides TRF counts as raw data which are not altered. Images can be optimized and stored in SoftMax® Pro Software. Raw counts can also be directly exported by the software to an integrated Excel macro spreadsheet for analysis and quantification of protein. Alternatively, data can be directly exported to ImageJ for analysis.
Sensitivity and dynamic range
The sensitivity and dynamic range of the system were tested using glutathione S-transferase (GST). A three-fold serial dilution of GST in 1x running buffer was loaded on a 4-20% gradient gel and run for 30 minutes. Proteins were transferred to an Immobilon FL membrane and probed with biotin labeled rabbit anti-GST for 2 hours followed by incubation with ScanLater Eu-labeled streptavidin for 1 hour. The blot was washed, dried and scanned using SpectraMax Paradigm reader (Figure 2). The system demonstrated sub-picogram detection limit of GST with over 4 logs of positive response of the signal vs. amount of GST (Figure 3).
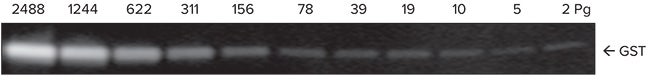
Figure 2. Image of GST dilution series as scanned by SpectraMax Paradigm reader.
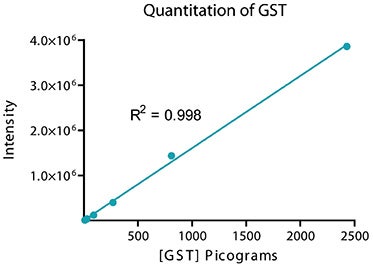
Figure 3. Integrated intensities from individual bands shown in Figure 2 analyzed with the SoftMax Pro integrated Excel macro showing total dynamic range of 4 logs and a linear dynamic range of 3 logs.
Signal stability
An exceptional feature of the Eu-labels is signal stability and resistance to photo bleaching. Blots can be scanned later as the signal is stable for months. (Figures 4 and 5).

Figure 4. Eu-label signal stability and resistance to photo bleaching.
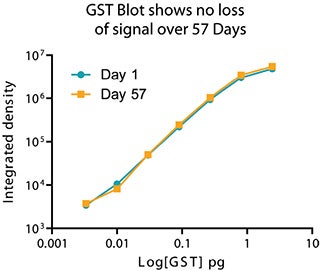
Figure 5. Plot of mean intensity vs. picograms of GST illustrates signal stability after 57 days.
Blots can be scanned multiple times without loss of signal
Serial two-fold dilution of transferrin in 1 x sample buffer was loaded on a 4-20% gradient gel and run for 30 minutes. Proteins were transferred to Immobilon FL and probed with rabbit anti-transferrin for 2 hours, followed by probing with Eu-labeled anti-rabbit IgG for 1 hour. Blots were washed, dried and scanned seven times in a row using a SpectraMax Paradigm reader (Figure 6).
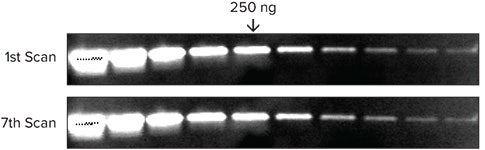
Figure 6. Signal is very stable and reproducible even after scanning multiple times.
Comparison to chemiluminescence western blot
HEK293T cells were treated with 0, 50, and 100 ppm of methyl methanesulfonate (MMS), a carcinogen that causes DNA damage. The extracts (80 μg) were run on a gel, and blots were probed with mouse anti-Rad18 followed by either ScanLater Eu-labeled anti-mouse antibody or HRP labeled anti-mouse antibody for the chemiluminescent assay (Immobilon Western Chemiluminescent HRP Substrate, Millipore cat. no. WBKLS 0500).
Rad18 is a protein that plays an essential role in DNA damage repair. It is present in two forms, ubiquitinated and nonubiquitinated. Shown in Figure 7, exposure to MMS decreases the amount of ubiquitinated Rad18 and increases the amount of non-ubiquitinated Rad18. Quantitation of the ScanLater blot was done using SoftMax Pro Software to capture the signal and export directly to an Excel macro for analysis. An Alphainnotech chemiluminescent imager was used to capture signal from the chemiluminescent blot. Quantitative analysis of the ScanLater blot shown in Figure 8 shows an increase in Rad18 protein and decrease in ubiquitinated Rad18 corresponding to increasing levels of MMS. This correlation was less clear using the chemiluminescent method.
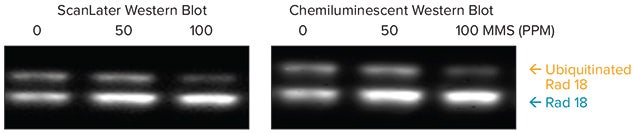
Figure 7. Comparison of detection of endogenous and ubiquitinated forms of Rad18, using ScanLater Western Blot and Chemiluminescent Systems.
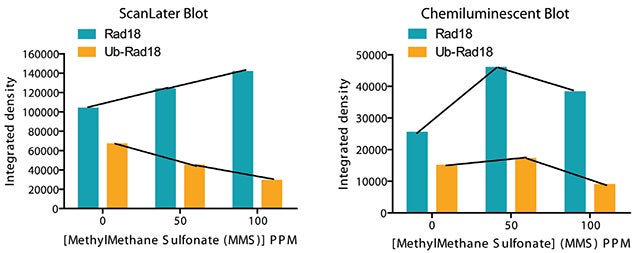
Figure 8. Scan later clearly detects a quantitative increase in the amount of unmodified Rad18 and decrease in the amount of ubiquitinated Rad18 with increase in the level of MMS. The data shows that the ScanLater TRF Western Blot is as sensitive as and more precise than the chemiluminescent blot.
Conclusion
Using ScanLater western blot technology, users can follow the workflow that is optimized for their application. Secondary antibody labeled with Eu enables low background detection in TRF mode on a SpectraMax i3 or SpectraMax Paradigm Multi-Mode Microplate Reader. Images are stored in SoftMax Pro Software which provides integrated export to a custom Excel macro or ImageJ for analysis.
ScanLater Western Blot Detection System provides quantitation of protein with a dynamic range of 4 logs and a linear range of 3 logs. In addition, the signal is stable over time and over multiple scans. ScanLater Western Blot Detection System is a simple, sensitive, and stable platform that provides excellent protein analysis capability in a multi-mode microplate reader.
简介
今天在制药和临床研究领域中对某种蛋白 进行检测是整个流程中重要环节, Western Blot(蛋白免疫印迹技术)也是 最常见的蛋白检测手段,多种技术方法都 可以对膜上的蛋白质进行定量检测,包括 荧光法和化学发光法。然而,每种技术手 段都有自身的局限性,尤其目前多数研究 都急需提高蛋白定量的准确性和动态学范 围。这里我们介绍一种全新技术用于 Western Blot检测的系统,此系统所需检 测仪器为SpectraMax® i3和SpectraMax® Paradigm多功能微孔板检测系统。此篇 介绍文章中我们将展示其优势,包括降低 背景噪音后带来的超宽动态学范围、以及 具有多次检测后依然稳定不变的信号强度 值。此外,对比与传统发光检测方式, Scanlater优化了其检测的灵敏度。
此方法也不涉及对酶分子的检测,同时也 利用了铕元素很强的抗光漂白特性。因 此,信号在数星期和数月内均稳定可检 测。稳定的信号输出可以多次重复检测该 膜均可获得一致精确的结果。全新的 ScanLater Western Blot检测系统简单、 灵活、灵敏,是基于微孔板检测平台之上 的蛋白定量检测分析系统。
检测试验
ScanLate® Western Blot检测系统流程与 传统经典方式类似,包括制胶、转印直到 二抗孵育,将转印蛋白的膜与铕元素标记 的二抗或铕元素标记的生物素进行孵育, 这些二抗可以与特异性的一抗结合,而这 些一抗可与膜上感兴趣的蛋白相互结合(图 一);

图一:ScanLate® Western Blot检测流程(1) 一抗识别膜上蛋白(2)铕元素标记的二抗与一抗结 合(3)结合二抗通过ScanLater TRF Western Blot检测卡盒进行扫描成像。
成像利用铕元素具有1ms荧光半衰期的时 间分辨荧光(TRF)特性进行检测,这样可 以大大降低背景自发光和其它短半衰期荧 光信号的干扰,显著降低背景信号值。如 此方式不会出现化学发光和普通荧光检测 时的过曝现象,所以通过此系统进行 Western Blot检测时可获得更加锐利的条 带和更高的成像质量。
SoftMax Pro 软件分析 Western Blot数据
以下所有实验数据均出自于ScanLater Western Blot与SpectraMax i3或SpectraMax Paradigm多功能微孔板检测系统整合后, 利用了微孔板检测系统这个平台,使其具 有了许多独一无二的优势。首先,数字光 子计数模式使得TRF原始信号值无需转 换。成像结果可以存储于SoftMax Pro软 件中直接进行优化分析。原始计数结果也 可以直接输出至Excel表格中进行分析和 定量处理。或许直接输出至Image J软件 中进行分析。
灵敏度和动态学范围
灵敏度和动态学范围的检测利用了谷胱甘 肽转移酶(GST)作为研究对象,三倍体系 的梯度稀释于1X缓冲液中,上样于4-20% 的梯度胶中加电压分离30分钟。蛋白转印 至荧光膜上后用生物素标记的兔抗GST探 针分子进行孵育2小时,然后在与铕元素 标记的链霉亲和素孵育1小时,膜清洗干 燥后,使用SpectraMax Paradigm微孔板 检测平台进行检测(图二)。系统检测结果 展示其亚皮克级别检测灵敏度,超过4数 量级的动态学范围(图三)。

图二:GST梯度稀释后利用SpectraMax Paradigm检测结果。

图三:整和单独条带的信号强度值,将数据导出至Excel进行分析获得4个数量级动态学范围,线性范围可达3个数量级。
信号稳定性
铕元素最特有的优势在于信号稳定性高和抗光漂白性强。膜放置数月后进行检测信号值依然稳定(图四和图五)。

图四:铕元素标记后信号稳定性高和抗光漂白性强。

图五:以荧光平均强度值Vs皮克级别的GST含量作图比较,信号强度在57天后依然稳定。
多次膜扫描检测后信号强度无损失
转铁蛋白经过两倍连续稀释于1X样品缓冲 液后上样于4-20%梯度胶后稳压30分钟。 转印目标蛋白于荧光膜上,使用兔抗转铁 蛋白一抗孵育2小时,随后使用铕元素标 记的抗兔IgG孵育1小时。膜清洗、干燥和 使用SpectraMax Paradigm多功能微孔板 检测平台重复七次进行扫膜(图六)。

图六:经过多次扫膜后信号强度稳定、重复检测信号值不变。
与化学发光Western Blot法比较
用0,50和100ppm不同浓度的甲基磺酸甲 酯(MMS),即是一种致癌物质可导致DNA 损伤,处理HEK293T细胞。抽提(80ug)细 胞蛋白跑胶,转膜后使用鼠源anti-Rad18 抗体进行孵育,二抗分别选用铕元素标记 的抗鼠抗体或辣根过氧化物酶标记的抗鼠 抗体(HRP化学发光底物选用Millipore产 品 cat.on.WBKLS0500)。
已知Rad18是在DNA损伤修复过程中发挥 作用的重要一种蛋白,目前有两种形式存 在,泛素化和非泛素化形式。如图七所示 经过MMS处理后的细胞其泛素化的Rad18 含量降低而非泛素化的Rad18含量明显增 加。荧光检测时使用SoftMax Pro软件收 集膜上信号后直接输出信号至Excel中进 行分析,化学发光时使用Alphainnotech 化学发光成像设备检测膜上信号。 ScanLater膜定量分析结果如图八所示, 清洗分辨出随着MMS含量增加其Rad18蛋 白增加和泛素化Rad18蛋白的减少,这种 相关变化一目了然。

图七:比较两种方式进行内源性泛素化形式的Rad18蛋白的检测结果如上图所示。

图八:使用ScanLater方式可检测出伴随着MMS梯度增加,其泛素化Rad18蛋白含量规律性的降低和非泛素化Rad18含量规律性的增加, 可以发现ScanLater方法获得检测结果与经典化学发光方法比较具有一样的灵敏度,但是表现出更高的精确度。
结论:
本文中介绍的ScanLater Western Blot技 术,用户可以按照如下流程来优化自己的 应用,其次,铕元素标记的二抗因为具有 时间分辨荧光优势特点即其大大降低背景 噪音,当在SpectraMax i3 或SpectraMax Paradigm多功能微孔板检测平台上进行 检测时,通过仪器自带的SoftMax Pro 软 件可以方便导出Excel或ImageJ中进行分 析。
ScanLater Western Blot检测技术可以在 蛋白定量检测时达到4个数量级的动态学 范围,线性动态学范围可以达到3个数量 级,多次扫描后其信号不变。所以我们可 以发现ScanLater Western Blot系统是一 种简单、灵敏、稳定性高的检测平台,可 以在基于多功能微孔板检测系统上具有出 色的蛋白定量分析能力。