
Application Note
Dual-Luciferase Reporter (DLR) Assay on the SpectraMax i3x Multi-Mode Microplate Reader with SpectraMax Injector Cartridge
- High-sensitivity quantitation of luciferase activity with 6-decade linear range
- Automatic data analysis and calculation of normalized reporter activity in SoftMax Pro Software
- SmartInject™ Technology for reagent mixing with optimal results
Introduction
Reporter gene assays are used to study the expression of eukaryotic genes. In dual reporter gene assays, cells are transfected with two vectors, the first containing an experimental reporter gene coupled to a regulated promoter of interest, and the second containing a control reporter gene coupled to a constitutive promoter. Normalizing the activity of the experimental reporter to the control reporter minimizes experimental variability.
Bioluminescent reporter systems using firefly and Renilla luciferases are widely used as co-reporters because both assays are easy to run and exquisitely sensitive. Promega’s Dual-Luciferase® Reporter (DLR) Assay System allows users to measure both firefly and Renilla luciferase activity in a single microplate well, with firefly acting as the experimental reporter and Renilla the control. Figure 1 illustrates the two enzymatic reactions, which take place sequentially within the same assay well. Firefly luciferase enzyme catalyzes the oxidation of luciferin with the concomitant release of light.1 The reaction requires ATP, Mg2+ and O2. Renilla luciferase catalyzes the O2-dependent oxidation of coelenterate luciferin (coelenterazine) but does not require Mg2+ or ATP.2 The enzymes have different substrate requirements, so they can both be measured in a single reaction mixture.
The DLR assay requires delivery of two separate reagents containing the different substrates, each followed by a luminescence read. This assay workflow is easily performed using the SpectraMax i3x Multi-Mode Microplate Reader with SpectraMax Injector Cartridge, which is fully DL Ready validated.3 In this application note we demonstrate a linear dynamic range of 6 decades for recombinant firefly and Renilla luciferases, as well as linear detection of luciferase-transfected cells from 195 to 25,000 cells per well.
Materials
- Dual-Luciferase Reporter Assay System (Promega cat. #E1960); contents include:
- Luciferase Assay Buffer II
- Luciferase Assay Substrate
- Stop & Glo Buffer
- Stop & Glo Substrate
- 5X Passive Lysis Buffer
- Purified recombinant luciferase enzymes:
- Firefly luciferase: QuantiLum ® Recombinant Luciferase (Promega cat. #E1701)
- Renilla luciferase: Recombinant Renilla Luciferase (RayBiotech cat. # RB-15-0003P-10)
- CHO-K1 cells (ATCC cat. #CCL-61)
- Control luciferase vectors:
- pGL4.13[luc2/SV40] firefly luciferase vector (Promega cat. #E6681)
- pGL4.74[hRluc/TK] Renilla luciferase vector (Promega cat. #E6921)
- Fugene HD Transfection Reagent (Promega cat. #E2311)
- 6-well tissue culture plates (Corning cat. #3516)
- 96-well flat clear bottom white TC-treated microplates (Corning cat. #3610)
- BrightMax sealing films (Genesee cat. #12-639)
- White 96- and 384-well microplates (Greiner cat. #655075 and #781075)
- SpectraMax i3x Multi-Mode Microplate Reader
- SpectraMax Injector Cartridge
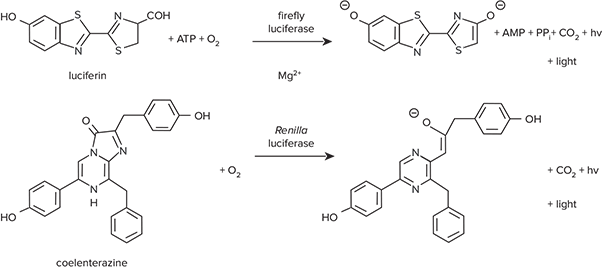
*Figure 1. Reactions catalyzed by firefly and *Renilla luciferases. Firefly and Renilla luciferase have different substrate requirements.
Methods
Enzyme standard curves
A stock solution of firefly luciferase was prepared by diluting the 12.4 mg/mL solution provided to 1 mg/mL with 1X Passive Lysis Buffer (PLB, a component of the Dual-Luciferase Reporter Assay System) containing 1 mg/mL BSA. Stock Renilla luciferase was prepared by reconstituting the lyophilized enzyme with 1X PBS to a concentration of 1 mg/mL.
Working solutions (10 µg/mL) of firefly and Renilla luciferases were made by transferring 10 µL of stock solution (1 mg/mL) into 990 µL PLB. A combined luciferase stock standard (100 ng/mL each) was then prepared by transferring 10 µL of each luciferase working solution into 980 µL PLB. Serial 1:10 dilutions of the combined stock standard yielded standards with concentrations ranging from 100 fg/mL to 100 ng/mL (1.6 fM to 1.6 nM). Luciferase Assay Reagent II (LAR II) and Stop & Glo Reagent were prepared according to the Dual-Luciferase Reporter Assay System technical manual.
Injector 1 of the SpectraMax Injector Cartridge was primed with 260 µL of LAR II, and Injector 2 was primed with 260 µL of Stop & Glo Reagent. In SoftMax Pro, the Acquisition View was used to configure the plate read with injection (Figure 2). Both injectors were set to deliver 100 µL (96-well plate) or 25 µL (384-well plate) of reagent using SmartInject Technology, which combines injection with plate shaking for complete reagent mixing, followed by a 2-second delay and 10-second integration. 20 µL (96-well plates) or 10 µL (384-well plates) of each luciferase standard was pipetted into assay wells. The assay plate was placed in the plate drawer of the SpectraMax i3x reader, and the read was initiated. Data analysis and graphing were performed using SoftMax Pro Software. A preconfigured Dual-Luciferase Reporter Assay protocol is included in the SoftMax Pro protocol library.
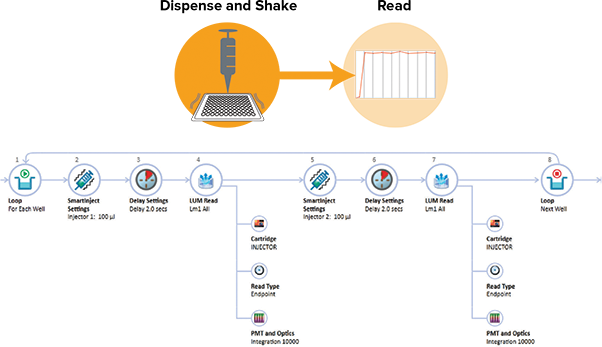
Figure 2. Acquisition Plan for DLR. The Acquisition Plan editor in SoftMax Pro enables a drag-anddrop setup of operations to be applied to each well. Shown above is the setup for the DLR assay. Two separate inject and read steps were applied. SmartInject, depicted in the graphic above the sample Acquisition Plan, enables shaking during the injection and 2-second delay step so that reagents are mixed thoroughly and signal develops quickly and consistently from well to well.
Cell-based assay
CHO-K1 cells were plated at 2.5x105 cells per well in 6-well culture plates and allowed to attach and grow overnight. The next day cells were transiently transfected with pGL4.13[luc2/SV40] firefly luciferase vector and pGL4.74[hRluc/TK] Renilla luciferase vector following a standard protocol for the Fugene HD transfection reagent. A ratio of 10:1 firefly: Renilla vector DNA was used, and each well received a total of 1 µg DNA and 3 µL Fugene HD transfection reagent.
24 hours after transfection, cells were seeded in a 96-well flat clear bottom white TC-treated microplate at densities from 195 to 25,000 cells per well and allowed to grow overnight. They were then lysed with 1X Passive Lysis Buffer and assayed in the same plate using the Dual-Luciferase Reporter Assay System and SpectraMax i3x reader with injector cartridge as described above. Prior to assay, a solid white plate seal was applied to the bottom of the microplate to maximize detection of luminescence signal.
Results
Luciferase standard curves
Signal for firefly and Renilla luciferase was linear across the 6-decade range of enzyme tested, from 1.6 fM to 1.6 nM (Figure 3). For the 96-well plate, this equates to 1 fg per well to 1 ng per well; for the 384-well plate it is 0.5 fg per well to 0.5 ng per well. Both 96- and 384-well assay formats yielded similar linearity and dynamic range, indicating the suitability of the dual-luciferase assay and SpectraMax i3x system for both formats.
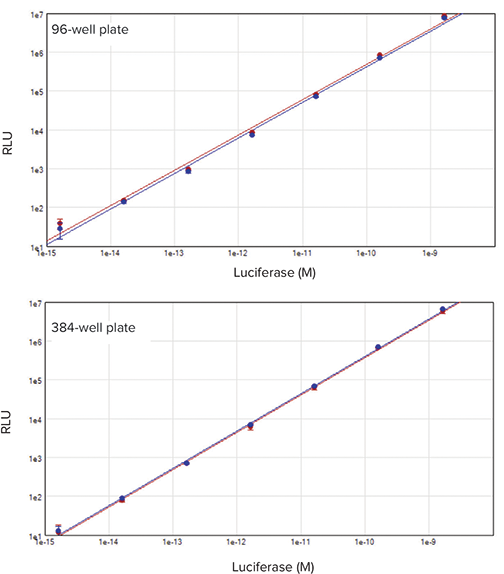
Figure 3. 96- and 384-well dual-luciferase standard curves. A 1:0 dilution series of purified, recombinant firefly (red plot) and Renilla (blue plot) luciferases was assayed using the DLR system. A linear range of 6 decades was measured (r2 = 0.994). Top, 96-well format; bottom, 384-well format.
Cell-based assay
Linearity of signal for both firefly and Renilla luciferases was observed across a broad range of cell densities, from 195 to 25,000 cells per well (Figure 4). The difference in magnitude of the luminescence signal between the two enzymes is due to the 10:1 ratio of firefly: Renilla vector used to transfect the cells, as well as the different strengths of the SV40 (firefly vector) and TK (Renilla vector) promoters.
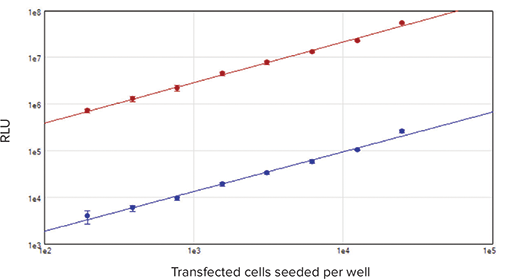
Figure 4. Cell-based standard curves. Cells transfected with both firefly and Renilla luciferases were seeded at densities from 195 to 25,000 cells per well in a 96-well plate and assayed using the DLR system. Results were plotted as RLU vs. number of transfected cells seeded per well. Red plot, firefly luciferase signal; blue plot, Renilla luciferase signal (r2 = 0.99 for both).
Figure 5 shows the firefly luciferase RLU values normalized to Renilla luciferase RLUs. The normalized values were similar across the entire range of cell densities tested.
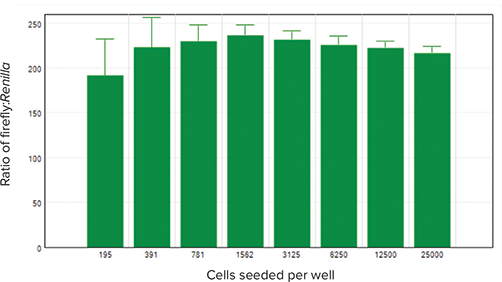
*Figure 5. Firefly luciferase signal in transfected cells was normalized to *Renilla luciferase signal and the resulting normalized values graphed vs. number of cells seeded per well.
Conclusion
The results above demonstrate excellent sensitivity, down to < 1 fg per well for firefly and Renilla luciferases, and a 6-decade dynamic range, ensuring accurate measurement of luciferase signal over an extensive range of luciferase expression levels and cell densities.
The SpectraMax i3x reader features a cooled photomultiplier tube (PMT) for low background noise in luminescence. When used in combination with the SpectraMax Injector Cartridge, this system enables a wealth of flash type luminescence applications including reporter gene and other assays with exceptional sensitivity and dynamic range. Analysis is done rapidly using a preconfigured SoftMax Pro protocol that displays each luciferase value and calculates normalized ratios for easy interpretation of results.
References
- DeLuca, M.A. and W.D. McElroy (1978) in Meth. Enzymol., 53:3.
- Matthews, J. C. et al. (1977). Purification and properties of Renilla reniformis luciferase. Biochemistry, 16:58.
- https://www.promega.com/products/pm/ dlready-luminometers/dlready-validatedluminometers
Learn more about SpectraMax i3x Multi-Mode Detection Platform >>